Study sheds light on the paths leading to the degradation of layered Li-rich oxide cathodes
In recent years, researchers have been trying to develop increasingly advanced battery technologies that can store more energy, recharge faster, discharge slower, and have longer lifespans. To achieve this, many have been experimenting with new cathode materials, as these tend to contribute significantly to a battery’s performance.
Layered lithium-rich transition metal oxides have recently become the focus of numerous research studies, as they have been found to be promising cathode materials. As cathode materials, they could theoretically help to boost the energy density of rechargeable batteries for both electric vehicles and portable devices.
The advantages of layered lithium-rich metal oxide cathodes derive from their layered structure and their composition. Their structure allows lithium atoms to move across layers while the battery is operating, while their richness in lithium allows them to store and release more energy while charging/discharging.
Moreover, these cathodes contain transition metals such as manganese (Mn), cobalt (Co) or nickel (Ni) and oxygen anions, which can facilitate redox (reduction-oxidation) reactions within the batteries. These are the reactions that allow batteries to gain and lose electrons, thus contributing to their production of energy.
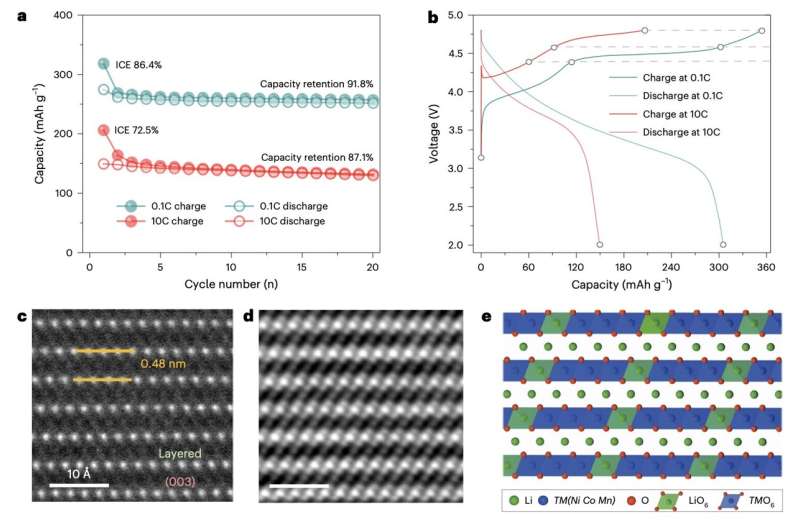
Despite their advantages, many layered lithium-rich metal oxide cathodes have been found to rapidly deteriorate and lose voltage over time. This, along with their instability, has so far prevented their large-scale use in battery development.
Researchers at Sichuan University, Southern University of Science and Technology in China and other institutes in various countries worldwide recently carried out a study investigating the pathways that lead to the degradation of these li-rich oxide cathodes.
Their paper, published in Nature Nanotechnology, outlines some of the structural, chemical, kinetic and thermodynamic effects that contribute to the reported short lifespans of batteries containing these cathodes.
“We integrate analyses of morphological, structural and oxidation state evolution from individual atoms to secondary particles,” Zhimeng Liu, Yuqiang Zeng and their colleagues wrote in their paper. “By performing nanoscale to microscale characterizations, distinct structural change pathways associated with intraparticle heterogeneous reactions are identified.”
The researchers closely examined what happened in the cathodes at nanoscale and microscale using various advanced imagining techniques. This included energy-resolved transmission X-ray microscopy (TXM), which allows researchers to visualize materials at a remarkably high resolution, while also gathering information about their structural and chemical composition.
Using TMX, the team identified various oxygen defects and distortions at different charging rates in the first cycle of operation. These defects were found to prompt degradation via various possible routes.
“The high level of oxygen defects formed throughout the particle by slow electrochemical activation triggers progressive phase transformation and the formation of nanovoids,” Liu, Zeng and their colleagues wrote.
“Ultrafast lithium (de)intercalation leads to oxygen-distortion-dominated lattice displacement, transition metal ion dissolution and lithium site variation. These inhomogeneous and irreversible structural changes are responsible for the low initial Coulombic efficiency, and ongoing particle cracking and expansion in the subsequent cycles.”
The recent study sheds new light on the structural and chemical factors underpinning the degradation of layered Li-rich cathodes over time. In the future, the findings gathered by this research group could inform the development of effective strategies to reduce or mitigate these factors, which may in turn facilitate the use of these cathodes in next-generation batteries.
More information:
Zhimeng Liu et al, Revealing the degradation pathways of layered Li-rich oxide cathodes, Nature Nanotechnology (2024). DOI: 10.1038/s41565-024-01773-4.
© 2024 Science X Network
Citation:
Study sheds light on the paths leading to the degradation of layered Li-rich oxide cathodes (2024, September 30)
retrieved 30 September 2024
from https://techxplore.com/news/2024-09-paths-degradation-layered-li-rich.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
Comments are closed