New device architecture enables streamlined production of formic acid from CO₂ using renewable electricity
Carbon dioxide (CO2) is both an essential resource for life on Earth and a greenhouse gas contributing to global warming. Today, scientists are investigating CO2 as a promising resource that can be utilized to create renewable low-carbon fuels and high-value chemical products.
The challenge for researchers has been to identify efficient and cost-effective CO2 conversion pathways to premium carbon intermediates, such as carbon monoxide, methanol, or formic acid.
A research team led by K.C. Neyerlin at the National Renewable Energy Laboratory (NREL), with members from Argonne National Laboratory and Oak Ridge National Laboratory, discovered a promising solution to this challenge. The team developed a conversion pathway to produce formic acid from CO2 with high energy efficiency and durability while using renewable electricity.
The study, titled “A scalable membrane electrode assembly architecture for efficient electrochemical conversion of CO2 to formic acid,” was published in Nature Communications.
Formic acid is a potential intermediate chemical with a wide range of applications, especially as a raw material for the chemical or biomanufacturing industries. Formic acid has also been identified as an input for biological upgrading into sustainable aviation fuel.
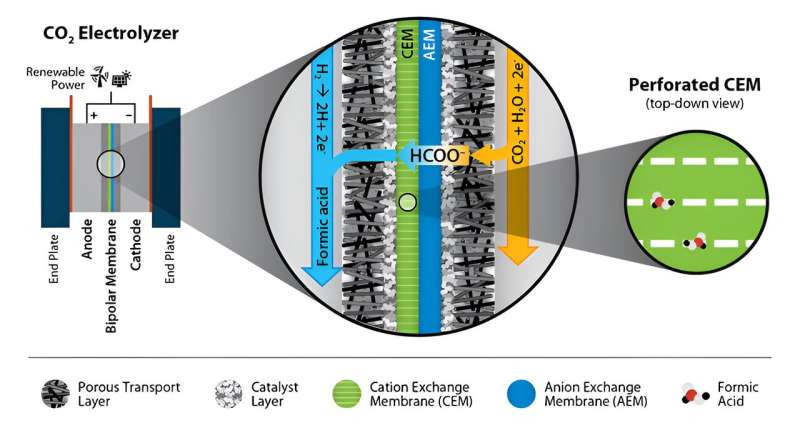
Novel perforated cation exchange membrane improves formic acid production
When an electrical potential is applied to an electrolyzer cell, CO2 electrolysis results in the reduction of CO2 into chemical intermediates such as formic acid or molecules such as ethylene.
The membrane electrode assembly (MEA) in the electrolyzer cell typically includes an ionically conductive membrane (cation or anion exchange) pressed between two electrodes consisting of electrocatalysts and ionically conducting polymers.
Leveraging the team’s experience in fuel cells and hydrogen electrolysis technology, they investigated several MEA configurations in an electrolyzer cell to compare electrochemical reduction of CO2 to formic acid.
Based on the failure analysis for the various designs, the team sought to exploit limitations in current material sets, specifically the lack of ion exclusion in today’s anion exchange membranes, and simplify the overall system design.
The resulting invention by NREL’s K.C. Neyerlin and Leiming Hu was an MEA electrolyzer modified with a novel perforated cation exchange membrane. This perforated membrane helped achieve stable, high-selectivity formic acid production, and it simplifies the design by using off the shelf components.
“The result of this study is a paradigm shift in the electrochemical production of organic acids like formic acid,” corresponding author Neyerlin said. “The perforated membrane architecture reduces the complexity of previous designs and can also be leveraged to improve energy efficiency and durability for other electrochemical CO2 conversion devices.”
Analysis confirms pathway economically viable at scale and with use of commercially available components
As with any research breakthrough it is important to understand the cost drivers and economic viability. Through a cross-directorate collaboration, NREL researchers Zhe Huang and Ling Tao provided a techno-economic analysis identifying pathways to cost parity with today’s industrial formic acid production processes when renewable electricity costs were at or below 2.3 cents per kWh.
“The team achieved these results with commercially available catalysts and polymer membrane materials, while producing an MEA design that leverages the scalability of today’s fuel cell and hydrogen electrolysis stacks,” Neyerlin said.
“The results of this study may enable a more rapid transition to scale and commercialization using renewable electricity and hydrogen to convert CO2 into fuels and chemicals.”
Electrochemical conversion technologies are a major pillar of NREL’s Electrons to Molecules initiative, which focuses on electricity-driven processes for a future generation of renewable hydrogen, net-zero fuels, chemicals, and materials.
“Our initiative is investigating pathways that use renewable electricity to convert molecules such as CO2 and water into compounds that can act as energy carriers and/or precursors for generating fuels or chemicals,” said Randy Cortright, NREL’s strategic lead for Electrons to Molecules.
“This electrochemical conversion research provides a breakthrough that can be utilized over a range of electrochemical conversion processes, and we look forward to additional promising results from this group.”
More information:
Leiming Hu et al, A scalable membrane electrode assembly architecture for efficient electrochemical conversion of CO2 to formic acid, Nature Communications (2023). DOI: 10.1038/s41467-023-43409-6
National Renewable Energy Laboratory
Citation:
New device architecture enables streamlined production of formic acid from CO₂ using renewable electricity (2024, May 15)
retrieved 15 May 2024
from https://techxplore.com/news/2024-05-device-architecture-enables-production-formic.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
Comments are closed