Hydrophobic surface coating strategy addresses CO₂ electroreduction stability challenges
The conversion of carbon dioxide (CO2) into valuable chemical products via the electrochemical CO2 conversion reaction could be highly advantageous. This conversion process could help to make good use of excess CO2 in the air collected by carbon capture solutions, thus potentially contributing to the reduction of pollution on Earth.
While past studies have observed salt formation, the underlying mechanism is not yet fully understood. Furthermore, despite the development of various salt removal solutions, effective strategies to prevent or eliminate salt precipitation without compromising the electrolyzer’s long-term stability remain elusive.
Their lack of stability is linked to the formation of bicarbonate salts at the negatively charged electrode (i.e., cathodes) in existing conversion technologies.
These salts can accumulate over time, ultimately blocking the gas flow channels and the gas diffusion electrode (GDE) backside. This blockage can in turn obstruct the flow of CO2 in devices, which can in turn significantly limit their efficiency.
Researchers at Rice University and University of Houston recently devised a strategy to track the formation of bicarbonate salts under different device operating conditions.
Using this approach, introduced in a paper published in Nature Energy, they were able to better understand how these salts are formed and introduce a fabrication process that could mitigate their formation.
“This work was inspired by our observation that although high concentrations of carbonate ions form at the catalyst/AEM interface owing to the high local pH during CO2RR, bicarbonate salt crystals predominantly precipitate on the GDE backside, not at the catalyst layer,” Haotian Wang, corresponding author of the paper, told Tech Xplore.
“The primary objective of our study was to understand the salt formation process and develop a strategy to mitigate salt precipitation in MEA-based CO2RR systems and extend device stability.”
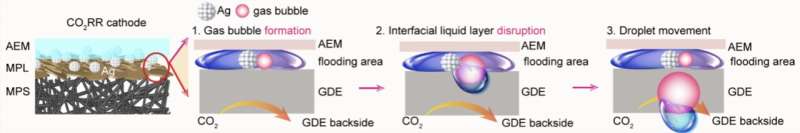
After closely examining the electroreduction of carbon dioxide under different conditions using advanced real-time monitoring techniques, the researchers observed a process resulting in the formation of bicarbonate salts.
Specifically, they observed the migration of liquid droplets containing positively charged ions and bicarbonate ions resulting from the release of gas at the electrode surface (i.e., interfacial gas evolution). When these droplets dried out, they were found to leave solid salt residues, which ultimately obstructed the flow of CO2 gas.
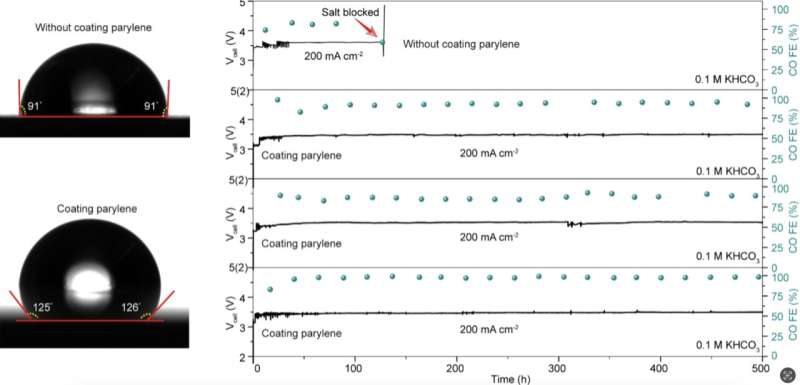
Drawing from this insight, they devised a fabrication strategy that could prevent the formation of these salt crystals. Their proposed strategy entails the application of a water-repelling polymer layer to the surface of the channel in which gas flows.
“We applied a hydrophobic parylene coating to the surface of the cathode gas flow channel in MEA electrolyzers to facilitate salt droplet removal,” explained Hao.
“One of our most notable findings was that observations of salt formation in CO2 reduction electrolyzers were used to propose a mechanism for salt precipitation linked to the drying of liquid droplets carrying cations and (bi)carbonate ions.”
In initial assessments, Hao and his colleagues found that their fabrication strategy significantly reduced the accumulation of droplets and subsequently salt crystals. This in turn improved the stability of a system for the electrochemical reduction of CO2, boosting the time for which it operated reliably from ~100 hours to over 500 hours at 200 mA/cm2.
The strategy proposed by this research team could soon be tested further and applied to other electrolyzers for the conversion of CO2, to improve their stability. This could contribute to the advancement of these technologies, which could facilitate their future large-scale deployment.
“A key achievement is that a hydrophobic surface coating was used to remove droplets from the flow channels before they could dry, increasing the operational stability of the electrolyzer,” added Hao.
“In our next studies, we will also explore whether CO2RR stability can be further enhanced by combining hydrophobic coating techniques with optimized gas diffusion electrode designs or alternative salt removal strategies.”
More information:
Shaoyun Hao et al, Improving the operational stability of electrochemical CO2 reduction reaction via salt precipitation understanding and management, Nature Energy (2025). DOI: 10.1038/s41560-024-01695-4.
© 2025 Science X Network
Citation:
Hydrophobic surface coating strategy addresses CO₂ electroreduction stability challenges (2025, February 19)
retrieved 19 February 2025
from https://techxplore.com/news/2025-02-hydrophobic-surface-coating-strategy-electroreduction.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
Comments are closed