Flexible pseudocapacitor defies climate extremes, packs energy punch
by Dr. Debasis Ghosh , Tech Xplore
In today’s rapidly evolving technological landscape, the demand for reliable energy storage solutions has never been greater. With our reliance on electronic devices growing, especially in aviation, space exploration, and satellite operations, the need for energy storage that can withstand extreme temperatures is paramount.
For decades, batteries have been the cornerstone of energy storage, offering high energy density but plagued by safety concerns and limited performance in extreme temperatures. Meanwhile, capacitors, known for their high power and durability, have struggled to match the energy density of batteries. Enter pseudocapacitors—devices that bridge the gap between batteries and capacitors, offering high power and energy density.
Driven by a curiosity to explore alternative solutions to traditional batteries, I embarked on a journey to unlock the potential of pseudocapacitors. The work is published in the journal Small.
Alongside my colleagues, Dr. Chirodeep Bakli (IIT Kharagpur, India) and Dr. Hyunyoung Jung (GNU, South Korea), we ventured into uncharted territory, pushing the boundaries of energy storage technology to power personal and portable flexible electronics in extreme climate conditions—say, at the top of Mount Everest or in Death Valley—with high energy/power density, utmost operational safety and ultralong cycle life.
With my Ph.D. scholar, Yadav Prahlad, I was developing vanadium oxide-based cathode materials for aqueous zinc ion batteries. We discovered that certain phase of vanadium oxide also shows high pseudocapacitance in aqueous-based electrolytes. However, the poor electrochemical stability window of aqueous-based electrolyte restricts the cell operation to ~1 V, which is too low to boost the energy density.
Upon switching from conventional salt-in-water to a water-in-salt-type electrolyte, we observed a significant improvement in operational voltage window (~2.5 V) and capacitance, leading to a high energy density. However, with only the water-in-salt electrolyte, which is a highly concentrated electrolyte, low-temperature operation is restricted due to the precipitation of salt at low temperature. But for practical applications, we were looking for an electrolyte that could support high operational voltage and be operated in any environment, including the coldest or hottest territories.
We presented a 2.5 V, all-climate operational pseudocapacitor constructed with laser-scribed, graphene-supported, electrodeposited vanadium oxides as electrodes and a novel water-in-salt hybrid electrolyte with high energy and power density, utmost operational safety and ultralong cycle life.
Our breakthrough came with the development of a novel electrode material: vanadium oxide (V5O12·6H2O) electrodeposited onto polyimide-film-derived, interdigital patterned, laser-scribed graphene, employing a CO2 laser. The device fabrication is quick, and the electrode architecture can be engineered at a small scale to suit applications in miniaturized electronics, too.
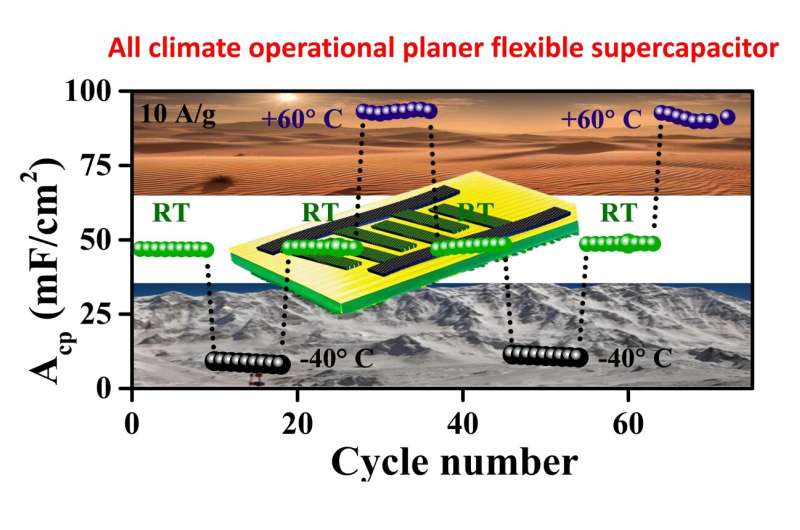
Complementing the electrode is a new water-in-salt hybrid (WISH) electrolyte, which extends the pseudocapacitor’s operating temperature range to an unprecedented -40° C to 60° C. The WISH electrolyte is composed of a high concentration (17 m) of sodium perchlorate salt dissolved in a solvent mixture of water and ethylene glycol. The ethylene glycol is a well-known anti-freezing agent to push the operational temperature limit to as low as -40°C.
The employed electrolyte is inflammable; thus, there are no fire hazards or thermal runway issues, which are often a problem with high-voltage devices employing organic electrolytes. Our pseudocapacitor showcases high energy and power performance with an areal capacitance of 234.7 mF/cm2 at room temperature, corresponding to a high energy density of 203.7 µWh/cm2.
Even at a bone-chilling -40°C, it maintains a formidable capacitance of 129.8 mF/cm2. Furthermore, after 80,950 charge-discharge cycles at room temperature, it retained 76% of its initial capacitance, highlighting its remarkable stability and durability. My colleague, Dr. Chirodeep Bakli from IIT Kharagpur, helped us to understand the interfacial interplay at the electrode-electrolyte interface at different temperatures.
The significance of this achievement goes beyond scientific advancement. It represents a paradigm shift in energy storage technology—one that prioritizes versatility, reliability and sustainability. From powering remote sensors in harsh climates to enabling renewable energy integration, flexible pseudocapacitors hold the key to a cleaner, more resilient energy future.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about Science X Dialog and how to participate.
More information:
Prahlad Yadav et al, A 2.5 V In‐Plane Flexi‐Pseudocapacitor with Unprecedented Energy and Cycling Efficiency for All‐Weather Applications, Small (2024). DOI: 10.1002/smll.202400975
Small
Dr. Debasis Ghosh is an Associate Professor at Center for Nano and Material Sciences, JAIN (Deemed to be University). He is also a visiting Professor at Gyeongsang National University, South Korea. His research interest is on different rechargeable batteries, supercapacitors and hydrogen production.
Citation:
Flexible pseudocapacitor defies climate extremes, packs energy punch (2024, May 8)
retrieved 9 May 2024
from https://techxplore.com/news/2024-05-flexible-pseudocapacitor-defies-climate-extremes.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
Comments are closed